Antidepressants and Teens: Understanding the FDA Black Box Warning
What parents and teens need to know
When a teen is struggling with depression, one of the treatment options may include an antidepressant, such as an SSRI (selective serotonin reuptake inhibitor). These medicines can be very effective, but many families have heard about the FDA “black box” warning related to suicide risk and feel understandably worried.
At New Chapter TMS, we want families to have the facts so they can make informed decisions. Here’s what research tells us:
Why the Black Box Warning Exists
In 2004, the FDA required all antidepressants to carry a strong warning label about possible increased suicidal thoughts or behaviors in children and teens. This came from early clinical trials where about 4% of young patients on antidepressants reported new suicidal thoughts or behaviors, compared to about 2% on placebo.
Important to know:
- No teen in those older trials died by suicide.
- The increase in risk was small (roughly 1–2 more teens per 100).1,2
- Many teens experienced real improvement in their depression.
Because of this small risk, guidelines recommend close monitoring when starting medication.
What Recent Research Adds
After the black box warning was added, doctors prescribed fewer antidepressants to teens—about a 20% drop. Unfortunately, during that same time, suicide rates in young people began to rise again after more than a decade of decline. 3,4
This shows a difficult reality: not treating depression also carries real risks. Untreated depression itself is one of the strongest risk factors for suicide.
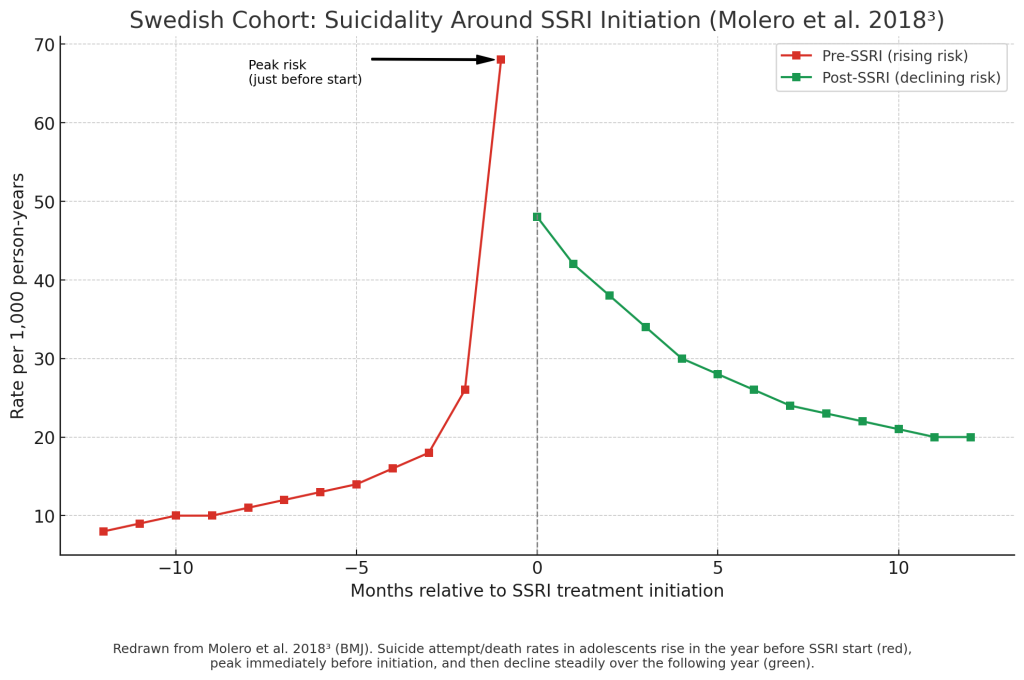
The Timeline of Suicide Risk and Antidepressants
Before starting medication:
Many adolescents begin treatment at their lowest point. Risk of suicidal thoughts or behaviors is already high before medication begins.5
The first few weeks of treatment:
This is the most important monitoring period. A small number of teens may feel more restless, agitated, or notice new suicidal thoughts soon after starting. Families and providers should check in often and watch closely for changes, 4,5
With ongoing treatment:
For most teens, antidepressants reduce suicidal thoughts and behaviors over time. Large studies show that risk steadily decreases after the first month on medication, especially as depression symptoms improve.2,5 By 6–12 months of treatment, the risk is much lower than it was before starting.
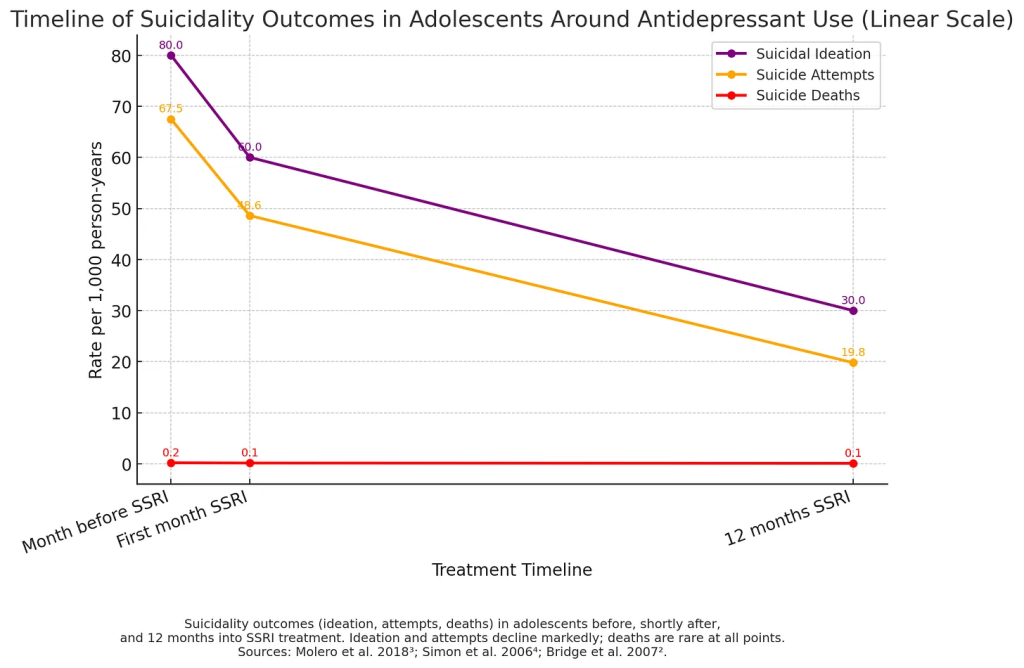
Thoughts, Attempts, and Deaths: Not All the Same
- Suicidal thoughts are relatively common in depressed teens.
- Suicide attempts are far less common.
- Suicide deaths are rarer still.
Research shows the increased risk associated with antidepressants is primarily related to thoughts, not deaths. Recent large studies show no evidence that antidepressants increase suicide deaths in teens; in fact, untreated depression carries the greater risk.
Balancing Risks and Benefits
The truth is that both depression and its treatment carry risks. But the evidence shows that:
- Untreated depression is one of the strongest predictors of suicide in adolescents.
- Antidepressants, when started carefully and monitored closely, reduce overall suicide risk in the long run. 2,5
- Most teens do better on medication than without it.
Doctors use the principle of “start low and go slow,” with frequent follow-ups in the first 1–2 months. Families play a critical role in providing support and noticing changes.
Bottom Line
Antidepressants are not risk-free, but for many teens with depression, they can be life-saving. The FDA black box warning is meant as a reminder to stay watchful—not to avoid treatment. With careful monitoring, therapy, and family involvement, the benefits usually outweigh the risks.
If you or your teen ever have thoughts of suicide, please know you are not alone. Help is available right away:
- If someone is in immediate danger, call 911.
- Call or text 988 to connect with the Suicide & Crisis Lifeline.
References
- Hammad TA, Laughren T, Racoosin J. Suicidality in Pediatric Patients Treated With Antidepressant Drugs. Arch Gen Psychiatry. 2006.
- Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment. JAMA. 2007.
- Gibbons RD, Brown CH, Hur K, Marcus SM, Mann JJ. Suicide risk and antidepressant drug development. Am J Psychiatry. 2007.
- Anderson KN, et al. Antidepressant Dispensing to US Adolescents and Young Adults, 2009–2022. Pediatrics. 2024.
- Li K, Zhou G, Xiao Y, et al. Risk of suicidal behaviors and antidepressant exposure among children and adolescents: A meta-analysis of observational studies. Front Psychiatry. 2022.
- Molero Y, Zetterqvist J, Binswanger IA, Hellner C, Larsson H, Fazel S. Antidepressant Use and Risk of Suicide and Attempted Suicide or Self Harm in People Aged 12 to 64: Cohort Study Using a Swedish Register-Based Cohort. BMJ. 2018;362:k2495.
- Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment. JAMA. 2007;297(15):1683–1696.
- Simon GE, Savarino J, Operskalski B, Wang PS. Suicide Risk During Antidepressant Treatment. Am J Psychiatry. 2006;163(1):41–47.
